|
Titanium position in the periodic table of elements
Mendeleev arranged the elements in the periodic table of elements by trying to coordinate the similar properties of the elements with the atomic masses. A quote of his: “I began to look about and write down the elements with their atomic weights and typical properties, analogous elements and like atomic weights on separate cards, and this soon convinced me that the properties of elements are in periodic dependence upon their atomic weights.” The structure he chose was of horizontal rows containing elements with increasing atomic weights, while trying to group elements with similar properties on the vertical columns. For a student, the first encounter with chemistry and the periodical table of elements can be a really scary experience. However there are smarter ways to study that can help achieve better grades! The pattern that emerged from this structure could only be achieved by leaving some places blank in some rows. This happened because at that time, the required elements were yet to be discovered. Mendeleev predicted that the discovery will happen in the future and even described some of the properties that each element would most likely display. The scientific research soon confirmed its prediction, the Mendeleev periodical table of elements soon gained universal recognition among established scientists. 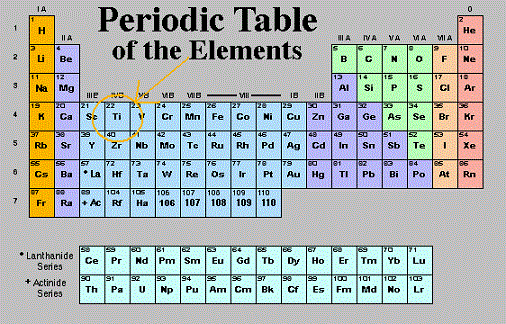
Titanium element is positioned in period 4, group 4 of the periodical table of elements, having an atomic number (atomic weight of 22). It is the occupies the first position in the block of transition metals, which is a common name for all the elements contained by the groups 4 to 12, with each element representing the successive addition of electrons to the "d" atomic orbital of the atoms, but without completely filling this shell.
Return from Periodic table of elements to Titanium Element Return to Titanium Home Page
|


